Thioredoxin A Is Essential for Motility and Contributes to Host Infection of Listeria monocytogenes via Redox Interactions - PubMed
doi: 10.3389/fcimb.2017.00287. eCollection 2017.
Zhimei Dong 1 , Xiao Han 1 , Hang Wang 1 , Li Jiang 1 , Jing Sun 1 , Yongchun Yang 1 , Tiantian Ma 1 , Chunyan Shao 1 , Xiaodu Wang 1 , Zhongwei Chen 1 , Weihuan Fang 1 2 , Nancy E Freitag 3 , Huarong Huang 4 , Houhui Song 1
Affiliations
- PMID: 28702378
- PMCID: PMC5487381
- DOI: 10.3389/fcimb.2017.00287
Free PMC article
Thioredoxin A Is Essential for Motility and Contributes to Host Infection of Listeria monocytogenes via Redox Interactions
Changyong Cheng et al. Front Cell Infect Microbiol. .
Free PMC article
Abstract
Microbes employ the thioredoxin system to defend against oxidative stress and ensure correct disulfide bonding to maintain protein function. Listeria monocytogenes has been shown to encode a putative thioredoxin, TrxA, but its biological roles and underlying mechanisms remain unknown. Here, we showed that expression of L. monocytogenes TrxA is significantly induced in bacteria treated with the thiol-specific oxidizing agent, diamide. Deletion of trxA markedly compromised tolerance of the pathogen to diamide, and mainly impaired early stages of infection in human intestinal epithelial Caco-2 cells. In addition, most trxA mutant bacteria were not associated with polymerized actin, and the rare bacteria that were associated with polymerized actin displayed very short tails or clouds during infection. Deletion or constitutive overexpression of TrxA, which was regulated by SigH, severely attenuated the virulence of the pathogen. Transcriptome analysis of L. monocytogenes revealed over 270 genes that were differentially transcribed in the ΔtrxA mutant compared to the wild-type, especially for the virulence-associated genes plcA, mpl, hly, actA, and plcB. Particularly, deletion of TrxA completely reduced LLO expression, and thereby led to a thoroughly impaired hemolytic activity. Expression of these virulence factors are positively regulated by the master regulator PrfA that was found here to use TrxA to maintain its reduced forms for activation. Interestingly, the trxA deletion mutant completely lacked flagella and was non-motile. We further confirmed that this deficiency is attributable to TrxA in maintaining the reduced intracellular monomer status of MogR, the key regulator for flagellar formation, to ensure correct dimerization. In summary, we demonstrated for the first time that L. monocytogenes thioredoxin A as a vital cellular reductase is essential for maintaining a highly reducing environment in the bacterial cytosol, which provides a favorable condition for protein folding and activation, and therefore contributes to bacterial virulence and motility.
Keywords: Listeria monocytogenes; host infection; motility; redox interaction; thioredoxins.
Figures
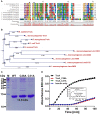
Cys28 and Cys31 of TrxA are required for oxidoreductase activity. (A) Amino acid sequence alignment of L. monocytogenes putative thioredoxins (TrxA, Lmo1609, 1903, 2152, 2424, and 2830) against homologs from B. subtilis, E. coli, P. aeruginosa, and S. aureus. Key amino acid residues denoted with asterisks are involved in catalytic activity. (B) Phylogenetic tree of L. monocytogenes putative thioredoxins and homologs from other bacterial species. The tree was constructed with the Neighbor-Joining (NJ) program and a bootstrap test of 100 replicates used to estimate the confidence of branching patterns, where the numbers on internal nodes represent the support values. (C) SDS-PAGE (12% gel) analysis of recombinant TrxA and its mutants, C28A and C31A. (D) In vitro insulin reduction assay of recombinant TrxA and its mutants, C28A and C31A. The inset shows the Michaelis-Menten plot and kinetic parameters, Km, Vmax, and kcat, for TrxA. Data are representative of three independent experiments.
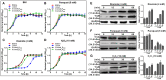
TrxA is responsible for bacterial resistance to thiol-specific oxidative stress. (A–D) Growth of L. monocytogenes wild-type EGD-e, mutant ΔtrxA and its two complement strains, CΔtrxA_Phelp and CΔtrxA_PtrxA, in BHI broth supplemented with or without oxidative agents. Overnight bacteria were re-suspended in fresh BHI alone (A) or BHI supplemented with 5 mM paraquat (B), 4 mM diamide (C) or 10 mM H2O2 (D), and incubated at 37°C for 12 h. Kinetic growth at OD600nm was measured at 1 h intervals. Data are expressed as means ± SD of three independent experiments. (E–G) Western blot analysis of TrxA expression under oxidative conditions. Total bacterial cell-free proteins were isolated at the indicated times after treatment with 4 mM diamide (E), 4 mM paraquat (F) or 10 mM H2O2 (G), and protein levels of TrxA and TrxB determined via western blot. GAPDH was used as the internal control. Results are indicated as a grayscale ratio of TrxA to GADPH or TrxB to GADPH. Data are expressed as means ± SD of three independent experiments.
TrxA contributes to L. monocytogenes motility and flagellar formation via interactions with MogR and GmaR (A,B). Motility assay (A) and transmission electron microscopy (TEM) (B) were performed on L. monocytogenes wild-type EGD-e, mutant ΔtrxA and its two complement strains, CΔtrxA_Phelp and CΔtrxA_PtrxA, grown on soft agar (0.25%) at 30° or 37°C. Mutant ΔflhB, which completely abolished bacterial motility, was taken as a reference control. (C–J) Isothermal titration calorimetry (ITC) measurement of protein-protein interactions. (C) ITC of interactions between MogR, the flagellar gene transcriptional repressor, and the anti-repressor, GmaR. (D) ITC of TrxA with MogR and GmaR. (E) ITC of TrxA binding to GmaR. (F) ITC of TrxA interactions with MogR. (G) ITC of interactions between the single mutant TrxA (C28A) and MogR. (H) ITC of interactions between the single mutant TrxA (C31A) and MogR. (I) ITC of interactions between the double Cys28/Cys31 mutant of TrxA (C28AC31A) and MogR. (J) ITC of interactions between the double Cys28/Cys31 mutant of TrxA (C28AC31A) and GmaR. For all titrations, raw data are presented in the upper panel and fitted binding curves in the lower panel.
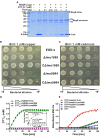
The redox state of MogR and enzymatic analysis and metal sensitivity assay. (A) The in vitro redox state of MogR changes under the efficient reduction by TrxA. MogR is reduced following incubation with the increasing amount of TrxA in the presence of DTT, and the redox state of MogR was visualized by SDS-PAGE and Coomassie blue staining. The reduced and oxidized forms of MogR are indicated as Re and Ox, respectively. (B,C) L. monocytogenes WT EGD-e, Δlmo1059, Δlmo0964 and the complement strains, CΔlmo1059 and CΔlmo0964, were subjected to copper (A) and cadmium (B) sensitivity assays by spotting 107 cfu of each bacterial strain onto BHI plates containing 1 mM copper and 1 mM cadmium. (D) The disulfide interchange reaction was used to determine the isomerase activities of TrxA, Lmo1059, and Lmo0964. The inset depicts the Michaelis-Menten plot and kinetic parameters, Km, Vmax, and kcat, for Lmo1059. (E) In vitro insulin reductase activity was assayed for recombinant TrxA, Lmo1059, and Lmo0964 over a period of 100 min. Data in (D,E) are expressed as means ± SD of three independent experiments.

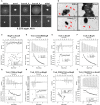
L. monocytogenes TrxA alters global gene expression profiles, especially regulating genes encoding virulence-associated factors. (A) Cluster analysis of differentially expressed genes in the WT and ΔtrxA mutant strains via two-dimensional hierarchical clustering. The red color indicates upregulation, green indicates downregulation and black indicates no changes, compared to wild-type. The X-axis represents samples while the Y-axis represents genes. (B) Clusters of orthologous groups (COG) classification for differentially expressed unigenes in the WT and ΔtrxA mutant strains. A total of 274 proteins were aligned to the COG protein database and classified functionally into 25 classes. Each function class is denoted by different capital letters under the x-axis. The y-axis represents the number of unigenes in a corresponding function class. (C,D) qRT-PCR validation of the transcriptome. Twenty-eight genes were randomly selected for qRT-PCR validation, including 16 representative upregulated genes (C) and 12 representative downregulated genes (D). The inset shows normalized relationships between qRT-PCR and transcriptomes of randomly selected genes. Values are presented as the log2 ratio (EGD-e/ΔtrxA) for genes. The coefficient of determination (r2) is indicated. All qRT-PCR data are expressed as means ± SD of three independent experiments. (E,F) TrxA is essential for LLO expression and hemolytic activity. Bacterial overnight cultures of L. monocytogenes strains were diluted into 100 mL fresh BHI and grown to the stationary phase. Bacterial pellets and cultures were collected to obtain the different cell fractions, as described in Section Materials and Methods. (E) Proteins were separated via 12% SDS-PAGE and immunoblotted with α-LLO or α-GAPDH antisera. The MW of each protein is indicated on the left. GAPDH was used as an internal control. (F) LLO-associated hemolytic activity was assessed by lysis of sheep red blood cells from serial dilutions of culture supernatants of bacterial strains. Data are expressed as means ± SD of three independent experiments. **P < 0.01.
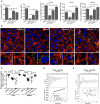
TrxA contributes to L. monocytogenes infection and virulence within mice. (A) Adhesion, invasion, and proliferation of L. monocytogenes in human intestinal epithelial cells, Caco-2. Cells infected with L. monocytogenes strains at the indicated time points were lysed and viable bacteria serially plated on BHI agar plates. The number of bacteria able to invade cells and survive are expressed as means ± SD of the recovery rate for each strain. *P < 0.05; **P < 0.01. (B) Actin tail formation in Caco-2 cells infected with L. monocytogenes strains 6 h post inoculation. Bacteria were detected with anti-Lm (green), bacteria actin tails and host actin using phalloidine (red), and cell nucleus labeled with DAPI (blue). Scale bar is 10 μm. The high-magnification images displayed at the bottom of each image show F-actin (red), bacteria (green), and nuclei (blue). (C) Proliferation of L. monocytogenes in mouse organs. Mice inoculated i.p. with L. monocytogenes strains (1 × 106 CFU) were euthanized 48 h after infection, and organs (liver and spleen) recovered and homogenized. Homogenates were serially plated on BHI agar. Numbers of bacteria colonized in liver and spleen are expressed as means ± SD of the recovery rate per organ for each group. *P < 0.05; **P < 0.01; ns means no significance. (D,E) The interactions analysis of TrxA (D) or the oxidized forms of TrxA (TrxAox) (E) with PrfA, the central virulence regulator of Listeria, by using the ITC assay. For all titrations, raw data are presented in the upper panel and fitted binding curves in the lower panel.

A proposed model for TrxA as a vital cellular reductase in L. monocytogenes. We propose that TrxA is essential for maintaining a highly reducing environment in the bacterial cytosol, which provides a favorable condition for protein folding and activation. Lmo0964 (DsbA) is responsible for direct oxidization of disulfide bonds in reduced MogR. Once MogR is correctly dimerized, the MogR:GmaR anti-repression complex is formed, which removes MogR from all flagellar motility gene promoters, enabling flagellar motility gene transcription at low temperatures. PrfA contains four cysteine residues in each monomer. TrxA contributes to maintain the reduced PrfA dimer that is exclusively activated in the cytosol in the presence of the co-factor GSH, and subsequently triggering expression of the virulence-associated factors.
Similar articles
-
Cheng C, Han X, Xu J, Sun J, Li K, Han Y, Chen M, Song H. Cheng C, et al. Gut Microbes. 2021 Jan-Dec;13(1):1-19. doi: 10.1080/19490976.2021.1884517. Gut Microbes. 2021. PMID: 33573432 Free PMC article.
-
Eshwar AK, Guldimann C, Oevermann A, Tasara T. Eshwar AK, et al. Front Cell Infect Microbiol. 2017 Oct 26;7:453. doi: 10.3389/fcimb.2017.00453. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 29124040 Free PMC article.
-
Ruhland BR, Reniere ML. Ruhland BR, et al. J Bacteriol. 2020 May 27;202(12):e00099-20. doi: 10.1128/JB.00099-20. Print 2020 May 27. J Bacteriol. 2020. PMID: 32253340 Free PMC article.
-
Grubaugh D, Regeimbal JM, Ghosh P, Zhou Y, Lauer P, Dubensky TW Jr,, Higgins DE. Grubaugh D, et al. Infect Immun. 2018 Feb 20;86(3):e00901-17. doi: 10.1128/IAI.00901-17. Print 2018 Mar. Infect Immun. 2018. PMID: 29263107 Free PMC article.
-
Pillich H, Puri M, Chakraborty T. Pillich H, et al. Curr Top Microbiol Immunol. 2017;399:113-132. doi: 10.1007/82_2016_30. Curr Top Microbiol Immunol. 2017. PMID: 27726006 Review.
Cited by 19 articles
-
Ma Z, Higgs M, Alqahtani M, Bakshi CS, Malik M. Ma Z, et al. J Bacteriol. 2022 May 17;204(5):e0008222. doi: 10.1128/jb.00082-22. Epub 2022 Apr 27. J Bacteriol. 2022. PMID: 35475633
-
Kho ZY, Azad MAK, Han ML, Zhu Y, Huang C, Schittenhelm RB, Naderer T, Velkov T, Selkrig J, Zhou QT, Li J. Kho ZY, et al. PLoS Pathog. 2022 Mar 1;18(3):e1010308. doi: 10.1371/journal.ppat.1010308. eCollection 2022 Mar. PLoS Pathog. 2022. PMID: 35231068 Free PMC article.
-
The Periplasmic Oxidoreductase DsbA Is Required for Virulence of the Phytopathogen Dickeya solani.
Przepiora T, Figaj D, Bogucka A, Fikowicz-Krosko J, Czajkowski R, Hugouvieux-Cotte-Pattat N, Skorko-Glonek J. Przepiora T, et al. Int J Mol Sci. 2022 Jan 9;23(2):697. doi: 10.3390/ijms23020697. Int J Mol Sci. 2022. PMID: 35054882 Free PMC article.
-
Cheng C, Han X, Xu J, Sun J, Li K, Han Y, Chen M, Song H. Cheng C, et al. Gut Microbes. 2021 Jan-Dec;13(1):1-19. doi: 10.1080/19490976.2021.1884517. Gut Microbes. 2021. PMID: 33573432 Free PMC article.
-
Luo ZH, Narsing Rao MP, Chen H, Hua ZS, Li Q, Hedlund BP, Dong ZY, Liu BB, Guo SX, Shu WS, Li WJ. Luo ZH, et al. Front Microbiol. 2021 Jan 8;11:608832. doi: 10.3389/fmicb.2020.608832. eCollection 2020. Front Microbiol. 2021. PMID: 33488549 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
-
Full Text Sources
-
Other Literature Sources
-
Medical
