Carboxyl-Terminal Residues N478 and V479 Required for the Cytolytic Activity of Listeriolysin O Play a Critical Role in Listeria monocytogenes Pathogenicity - PubMed
doi: 10.3389/fimmu.2017.01439. eCollection 2017.
Li Jiang 1 , Tiantian Ma 1 , Hang Wang 1 , Xiao Han 1 , Jing Sun 1 , Yongchun Yang 1 , Zhongwei Chen 1 , Huifei Yu 1 , Yi Hang 1 , Fengdan Liu 1 , Bosen Wang 1 , Weihuan Fang 1 2 , Huarong Huang 3 , Chun Fang 4 , Chang Cai 1 5 , Nancy Freitag 6 , Houhui Song 1
Affiliations
- PMID: 29163512
- PMCID: PMC5671954
- DOI: 10.3389/fimmu.2017.01439
Free PMC article
Carboxyl-Terminal Residues N478 and V479 Required for the Cytolytic Activity of Listeriolysin O Play a Critical Role in Listeria monocytogenes Pathogenicity
Changyong Cheng et al. Front Immunol. .
Free PMC article
Abstract
Listeria monocytogenes is a facultative intracellular pathogen that secretes the cytolysin listeriolysin O (LLO), which enables the bacteria to cross the phagosomal membrane. L. monocytogenes regulates LLO activity in the phagosome and minimizes its activity in the host cytosol. Mutants that fail to compartmentalize LLO activity are cytotoxic and have attenuated virulence. Here, we showed that residues N478 and V479 of LLO are required for LLO hemolytic activity and bacterial virulence. A single N478A mutation (LLON478A) significantly increased the hemolytic activity of LLO at a neutral pH, while no difference was observed at the optimum acidic pH, compared with wild-type LLO. Conversely, the mutant LLOV479A exhibited lower hemolytic activity at the acidic pH, but not at the neutral pH. The double mutant LLON478AV479A showed a greater decrease in hemolytic activity at both the acidic and neutral pHs. Interestingly, strains producing LLON478A or LLOV479A lysed erythrocytes similarly to the wild-type strain. Surprisingly, bacteria-secreting LLON478AV479A had barely detectable hemolytic activity, but exhibited host cell cytotoxicity, escaped from the phagosome, grew intracellularly, and spread cell-to-cell with the same efficiency as the wild-type strain, but were highly attenuated in virulence in mice. These data demonstrate that these two residues are required for LLO hemolytic activity and pathogenicity in mice, but not for escape from the phagosome and cell-to-cell spreading. The finding that the nearly non-hemolytic LLON478AV479A mutant grew intracellularly indicates that mutagenesis of a virulence determinant is a novel approach for the development of live vaccine strains.
Keywords: Listeria monocytogenes; cytolytic activity; listeriolysin O; live vaccine; virulence.
Figures
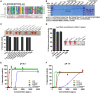
Residues N478 and V479 of listeriolysin O (LLO) are required for hemolytic activity. (A) The strategy for the generation of a mutated LLO with a 12-amino-acid in-frame deletion (472–GNARNINVYAKE–483) that could form an LPXTG motif. Stars indicate the identified key residues, N478 and V479, of LLO. The listed toxins are from the following organisms: LLO, L. monocytogenes listeriolysin; ALO, Bacillus anthracis anthrolysin; PFO, Clostridium perfringens perfringolysin; PLY, Streptococcus pneumoniae pneumolysin; SLO, Streptococcus pyogenes streptolysin; SLY, Streptococcus suis suilysin. (B) Sodium dodecyl sulfate–polyacrylamide gel electrophoresis analysis of the purified histidine-tagged recombinant LLO and its mutated forms expressed in Escherichia coli. (C,D) Comparison of the hemolytic activity of the LLO mutants relative to wild-type LLO. Erythrocytes incubated with 1% Triton X-100 or phosphate-buffered saline (PBS) served to determine the maximum (100%) and minimum (0%) hemolytic activity, respectively. (E,F) Hemolytic activity of the identified LLO mutants, LLON478A, LLOV479A, and LLON478AV479A, at various concentrations (0–4 ng/µL) at pH 5.5 (E) and 7.4 (F). Erythrocytes incubated with 1% Triton X-100 or PBS served to determine the maximum (100%) and minimum (0%) hemolytic activity, respectively. Data in C, D, E, and F are expressed as means ± SDs of three independent experiments.
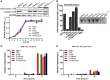
Listeria monocytogenes expressing LLON478AV479A lacks hemolytic activity but is cytotoxic to host cell membranes. (A) Secreted listeriolysin O (LLO) was detected by western blotting, and in vitro bacterial growth of the L. monocytogenes wild-type EGD-e and Δhly strains, and the complemented strains CΔhly, CΔhlyN478A, CΔhlyV479A, and CΔhlyN478AV479A. (B) Hemolytic activity of secreted LLO from the culture supernatants of the L. monocytogenes wild-type EGD-e and Δhly strains, and the complemented strains CΔhly, CΔhlyN478A, CΔhlyV479A, and CΔhlyN478AV479A. (C,D) Lactate dehydrogenase (LDH) release into the tissue culture medium was used to monitor the perforation of the host cell plasma membrane. The percentage of the maximal LDH release from monolayers of J774 macrophages infected with the indicated L. monocytogenes strains at 2, 4, and 6 h postinfection in the presence or absence of gentamicin (50 µg/mL) is indicated. All the data are expressed as means ± SDs of three independent experiments.

The N478V479 mutation of listeriolysin O reduces virulence in mice. (A) The Kaplan–Meier curve represents the survival of ICR mice over time. Ten mice in each experimental group were infected intraperitoneally with 1 × 107 CFU of Listeria monocytogenes and monitored for up to 7 days after infection. Data are represented as the percentage survival over time, and significance was determined via a log-rank test. (B) The L. monocytogenes wild-type EGD-e Δhly strains, and the complemented strains CΔhly, CΔhlyN478A, CΔhlyV479A, and CΔhlyN478AV479A were inoculated intraperitoneally into ICR mice at 5 × 106 CFU. Animals were euthanized 24 and 48 h after infection and organs (liver and spleen) were recovered and homogenized, and the homogenates were serially diluted and plated on brain–heart infusion (BHI) agar. The numbers of bacteria colonizing the liver and spleen are expressed as means ± SDs of the log10 CFU per organ for each group.
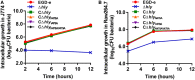
Intracellular growth of Listeria monocytogenes strains producing the indicated listeriolysin O proteins in murine-derived J774 and RAW264.7 macrophages. Gentamicin (50 µg/mL) was added 0.5 h postinfection. The J774 (A) and RAW264.7 (B) cells infected with the L. monocytogenes wild-type EGD-e and Δhly strains, and the complemented strains CΔhly, CΔhlyN478A, CΔhlyV479A, and CΔhlyN478AV479A were lysed at the indicated time points (2, 6, and 12 h), and viable bacteria were serially plated on brain–heart infusion agar plates. The number of recovered bacteria able to invade cells and survive are expressed as means ± SDs for each strain.

Listeria monocytogenes expressing LLON487V479 is not defective in cell-to-cell spreading. (A) Plaque sizes formed by the indicated L. monocytogenes mutants in L929 cell monolayers as a percentage of the plaque size formed by wild-type bacteria. Following bacterial internalization, gentamicin was added to a concentration of 10 µg/mL as indicated. The values shown represent the means of three independent experiments, and the error bars indicate the SDs. (B) Actin tail formation in Caco-2 cells infected with L. monocytogenes strains 6 h postinoculation. Bacteria were detected with anti-Lm (green), and bacteria actin tails and host actin were detected using phalloidin (red), while the cell nucleus was labeled with DAPI (blue). The scale bar is 10 µm. The high-magnification images displayed at the bottom of each image show F-actin (red), bacteria (green), and nuclei (blue).
Similar articles
-
Cheng C, Sun J, Yu H, Ma T, Guan C, Zeng H, Zhang X, Chen Z, Song H. Cheng C, et al. Front Immunol. 2020 Jun 3;11:1146. doi: 10.3389/fimmu.2020.01146. eCollection 2020. Front Immunol. 2020. PMID: 32582211 Free PMC article.
-
Glomski IJ, Gedde MM, Tsang AW, Swanson JA, Portnoy DA. Glomski IJ, et al. J Cell Biol. 2002 Mar 18;156(6):1029-38. doi: 10.1083/jcb.200201081. Epub 2002 Mar 18. J Cell Biol. 2002. PMID: 11901168 Free PMC article.
-
Glomski IJ, Decatur AL, Portnoy DA. Glomski IJ, et al. Infect Immun. 2003 Dec;71(12):6754-65. doi: 10.1128/IAI.71.12.6754-6765.2003. Infect Immun. 2003. PMID: 14638761 Free PMC article.
-
An Inducible Cre-lox System to Analyze the Role of LLO in Listeria monocytogenes Pathogenesis.
Nguyen BN, Portnoy DA. Nguyen BN, et al. Toxins (Basel). 2020 Jan 7;12(1):38. doi: 10.3390/toxins12010038. Toxins (Basel). 2020. PMID: 31936068 Free PMC article.
-
Mechanistic insights into ADXS11-001 human papillomavirus-associated cancer immunotherapy.
Miles BA, Monk BJ, Safran HP. Miles BA, et al. Gynecol Oncol Res Pract. 2017 Jun 2;4:9. doi: 10.1186/s40661-017-0046-9. eCollection 2017. Gynecol Oncol Res Pract. 2017. PMID: 28588899 Free PMC article. Review.
Cited by 4 articles
-
Chen R, Cheng RA, Wiedmann M, Orsi RH. Chen R, et al. mSphere. 2022 Feb 23;7(1):e0073021. doi: 10.1128/msphere.00730-21. Epub 2022 Jan 5. mSphere. 2022. PMID: 34986312 Free PMC article.
-
Cheng C, Han X, Xu J, Sun J, Li K, Han Y, Chen M, Song H. Cheng C, et al. Gut Microbes. 2021 Jan-Dec;13(1):1-19. doi: 10.1080/19490976.2021.1884517. Gut Microbes. 2021. PMID: 33573432 Free PMC article.
-
Cheng C, Sun J, Yu H, Ma T, Guan C, Zeng H, Zhang X, Chen Z, Song H. Cheng C, et al. Front Immunol. 2020 Jun 3;11:1146. doi: 10.3389/fimmu.2020.01146. eCollection 2020. Front Immunol. 2020. PMID: 32582211 Free PMC article.
-
Sun J, Hang Y, Han Y, Zhang X, Gan L, Cai C, Chen Z, Yang Y, Song Q, Shao C, Yang Y, Zhou Y, Wang X, Cheng C, Song H. Sun J, et al. Virulence. 2019 Dec;10(1):910-924. doi: 10.1080/21505594.2019.1685640. Virulence. 2019. PMID: 31680614 Free PMC article.
References
LinkOut - more resources
-
Full Text Sources
-
Other Literature Sources
